Drugs and vaccines for coronavirus. Current state of research

Coronavirus/ COVID- 19 still attacks. Another week of my self-isolation and working with offspring at my side has passed. I have started getting used to it, so maybe I shall not get crazy after all. Although you never know because from time to time I read internet or listen to the radio (I have not had TV for years now). Coronavirus pandemics everywhere. Sometimes there is news about drugs and vaccines for coronavirus but the impression one gets is that only a few companies are working on them. That’s why I decided to check myself what is the REAL and current state of research on drugs and vaccines for COVID-19. How many clinical studies are being conducted and where? And most of all: when are we going to see their results?
DRUGS AND VACCINES FOR CORONAVIRUS: 728 CLINICAL TRIALS
To start with, there are far more than just a few companies in the world are working on a COVID-19 medicine or vaccine. The most up-to-date data, from the 10th of April 2020 show that there are 728 clinical trials related to coronavirus being conducted globally at the moment.
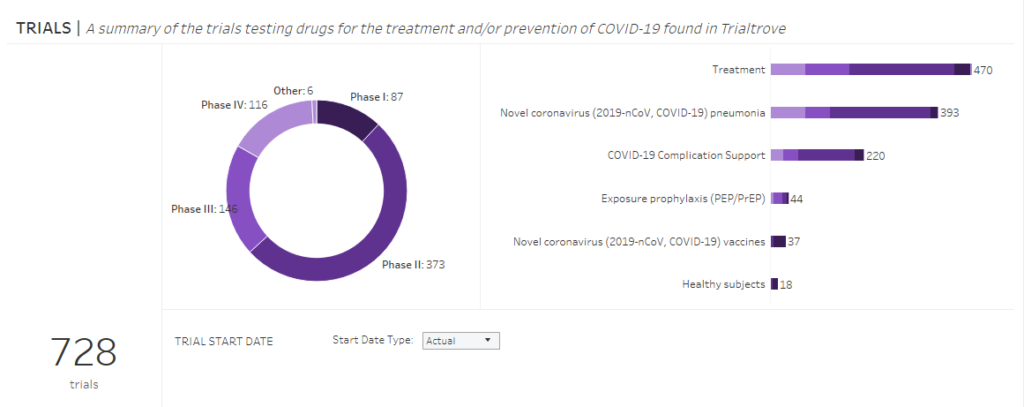
The number and the current status of clinical trials on drugs and vaccines for coronavirus globally. The status from the 10th of April 2020.
For comparison, only 6 days ago, on the 4th of April this was almost 300 trials less. Please check Polish version on this post from the 5h of April to find out yourself – the diagrams are in English.
New research and clinical trials are coming at a cosmic pace. Which is good news for you, me and the rest of humanity, of course.
ONLY 37 CLINICAL TRIALS ON THE COVID-19 VACCINE
Unfortunately, most studies are currently being conducted on the drug, not the vaccine. The data from the 10th of Aprils show that just 37 clinical studies being conducted now are vaccine tests.
Moreover, the overwhelming majority of the clinical trials over the vaccines have not gone beyond phase II clinical trials yet. Here, I only remind you briefly that healthy volunteers are involved in phase I and patients suffering from an illness participate in phase II and III of clinical studies.
Returning to the current state of research on drugs and vaccines for coronavirus, as you can see from the diagram above, we are much closer to the development of a drug than a vaccines.
This is mainly due to the fact that some anti-virus drugs which have been either in clinical trials or even already registered for use, are being tested now as potential cures for coronavirus. As you have noticed, the diagram above displays also phase IV clinical studies. These are so called “observational trials” during which one observes the effects of medicines which have been registered and approved for use already.
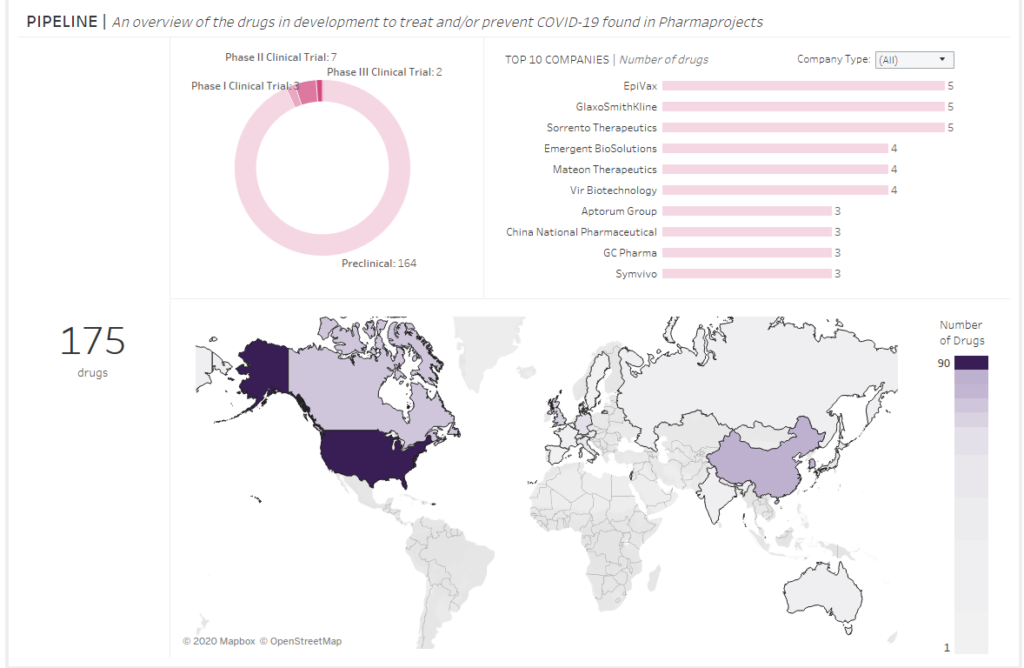
DRUGS AND VACCINES FOR CORONAVIRUS: WHO AND WHERE IS WORKING ON A DRUG?
Despite that there are 728 clinical trials on drugs and vaccines for coronavirus at the moment, only… 175 potential drugs have been reported so far. I please mind that 164 of them are still in a preclinical phase. This means they have not left laboratories yet (including test on animals).
Okay, so who and where is working on these drug for coronavirus??
The answer is quite easy to predict: primarily pharmaceutical companies from the USA and besides them also the Chinese, Canadians, the Japanese, the British (including GlaxoSmithKline) and very recently also Germans and Italians have joined the race for COVID-19 medicine or a vaccine.
Details in the diagram below:
DRUGS AND VACCINES FOR CORONAVIRUS: ARE THE CHINESE THE CLOSEST TO DEVELOP A DRUG FOR COVID-19 ?
All right, so numerous companies are working on dugs against coronavirus but who is the closest to succeed?
In my opinion: the Chinese. To be more specific, Ascletis Pharma Inc, a Hong Kong-registered biotechnology company which is the only one having a potential medicine in a phase III study. Again, please compare the diagram on the development of COVID-19 drugs included in the Polish version of this post.
Ascletis are exploring the possibility of treating patients with coronavirus with danoprevir, a drug which has proved to be effective in modern treatment of hepatitis type C.
In a nutshell: danoprevir prevents the multiplication of the virus causing hepatitis type C by preventing the translation of the genetic information of the virus into a specific protein structure. This means the virus cannot “connect” to the human body to multiply in it freely.
Here I say huge and laud “pardon” apologizing all experts on hepatitis type C and viruses if I have mixed something or simplified too much, just let me know in the comments.
Important that, danoprevir can make life difficult for the coronavirus just like the hepatitis wirusowi virus. Or at least that’s what the bosses of Ascletis Pharma Inc.say.

CAN THE RESEARCH ON DRUGS AND VACCINES FOR CORONAVIRUS
BE ACCELERATED FROM AN ORGANIZATIONAL PERSPECTIVE?
Conducting clinical trials on medicines and medical device is heavily regulated organizationally, ethically and legally.
For instance it is impossible to omit any phase of a clinical study over a new drug if one wants it be successfully approved for usage.
The question is: if in this a very unique situation, it would be possible to speed up somehow the clinical studies in COVID-19 drugs?
So far, the answer is not too much, but that can change.
On the 18th of March the International Coalition of Medicines Regulatory Authorities (ICMRA) held a virtual meeting of its members to discuss the course of COVID-19 related studies. Experts from the World Health Organization (WHO) , representatives of the European Commission and the European Medicines Agency (EMA) also joined this discussion. You can read the main points and conclusions of the deliberations yourself by clicking this link.
Experts were skeptical about skipping phases of clinical trials for coronavirus studies, although they were not against some simplification after all.
For instance if an animal testing proves promising, it can be allowed to start test on humans simultaneously, provided appropriate informed consents are obtained. The experts also suggested to perform the initial phase I clinical testing rather on younger volunteers.

CAN THE RESEARCH ON DRUGS AND VACCINES FOR CORONAVIRUS
BE ACCELERATED FROM A LEGAL PERSPECTIVE?
And how about the legal side of clinical studies? Maybe here one can count on some serious acceleration in legal assessment of the documentation required to issue a permission to register and start COVID-19 related study?
Well, the main problem is that there is no global clinical trial registration system (i.e. confirmation that the study has a positive ethical and legal assessment).
For now, every country in the world regulates the process of registering research on its own and in line with its applicable laws. The international regulations will not help us here.
For example, the Polish registration authority , i.e. the President of the Office for Registration of Medicines, Medical Devices and Biocidal Products (the President of the Office), communicated on the 23rd of March that the pandemics state would not affect the form of the proceeding, documentation or signature of documents submitted to the President of the Office. The regulations of Polish Act on Pharmaceutical Law and The Code of Administrative Procedure (CAP) will need to be observed – no changes here. To make it even more interesting, due to additional anti-coronavirus laws which have been processed by Polish Parliament and have become effective very recently, the procedure in front of the President of The Office can actually take longer than before the epidemics with none consequences for the Office as a representative of the public administration.
Luckily for the Poles, neither the provisions of the CAP, pharmaceutical law nor this new anti-coronavirus legislation prohibit the President of the Office to accelerate the assessment of the application for an administrative permission on a conduct of a clinical trial for a drug or vaccine for coronavirus.
And the President of the Office apparently intends to use such a possibility because in the communication of 17 March the Office asked all the documents related to COVID-19 studies are marked by adding the note “concerns the sars-cov-2 coronavirus” on the front page
The President stressed that such a designation “will help the Office’s staff to give priority to cases related to the SARS-CoV-2 coronavirus. “
Of course, this is just a declaration. But let’s stick to that.
As a consolation, I have not seen so far any really solid declarations on accelerating the approval process for a COVID-19 study from any country specific regulatory authority. US FDA has stated they will follow 24-hour-long revision and assessment of coronavirus related documents under their CTAP (Coronavirus Treatment Acceleration Program) but if they do not manage to do so, then, as far as I am aware, there will be no consequences.
We shall see what the future brings.
Best regards, Prawstoria.
P.S. When creating this post, I used publicly available data published and updated by the Pharmaintelligence.com an on-web service specializing in gathering and processing of information for the pharmaceutical industry. All reports I have used are free and publicly available. However, the materials are published only in English and Japanese.




